Hope in a vial
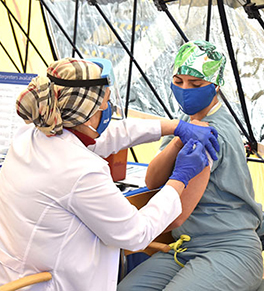
The UCI Health frontline caregivers receive their first COVID-19 vaccinations on Wednesday, Dec. 16. They will get a second shot in three weeks.
Photo by Carlos Puma for UCI Health
The first shipment of the Pfizer-BioNTech COVID-19 vaccine arrived at the UCI Medical Center Wednesday, Dec. 16, in specially designed deep cold storage packages, packed in dry ice to keep the temperature below about –70 degrees C (about –94 degrees F).
It was a moment workers at UCI Health had spent round-the-clock days prepping for.
Earlier in the week, they'd conducted drills to practice ferrying the vaccine into deep-freeze storage and reconstituting the vaccine from vials into injectable doses.
Within hours on Wednesday, the first doses were being injected into the arms of a handful of frontline UCI healthcare providers.
Hope at last
Wednesday’s injections were to be followed by a more expansive rollout for front-line clinical workers on Thursday. The shipment of 3,000 doses is enough to cover everyone in the first of five employee groups and some in the second group. By Sunday, UCI Health expects to have vaccinated 2,000 people.
Vaccinations started Wednesday with frontline clinical staff who care for patients in high-risk settings or patients who might have COVID-19.
They were joined by staff and physicians in the emergency department, intensive care unit (ICU) staff and physicians, clinicians who provide critical care, respiratory therapists, anesthesiologists and others in the priority first group.
“It’s been tested, and I trust the process,” says Dr. Cyrus Dastur, director of neurocritical care at UCI Health and among the first to receive the vaccine Wednesday. “I feel very comfortable getting one of the first shots available.”
“Hopefully we can begin to get past the pandemic next year and get back to a normal life,” adds Dastur, whose team treats incoming stroke patients — some of whom are COVID-positive — and provides neurological care for COVID patients.
He also helped with the vaccine rollout by participating on planning teams for UCI Health and the University of California Office of the President.
“I look forward to taking my children to see my parents without them having a risk of exposure. And I look forward to seeing the daily loss-of-life numbers go down. But it’s going to take some time for this to happen.”
All told, roughly 15,000 UCI Health employees are being offered the vaccine — any one of them who wants it is able to get it.
“More than 500 people signed up in the first hour after the vaccination schedule opened up on Monday afternoon, so our staff are clearly very excited to have this vaccine available,” UCI Health CEO Chad Lefteris says.
“Our teams have been working literally around the clock to prepare, receive, handle and distribute the vaccine to our employees.”
The first shipment of the Pfizer vaccine was expected to provide 3,000 UCI Health employees with the first in the two-dose series of inoculations. The Moderna COVID-19 vaccine, which is expected to arrive next week, will cover another 5,000 UCI Health employees.
Rollout challenges
UCI Health has been able to rely on its experience providing influenza vaccinations to thousands of staff members every year, but the Pfizer COVID-19 vaccine poses unique logistical challenges.
The vaccine has to be kept extremely cold and must be prepared according to specific requirements.
Consent forms must be collected from all patients. Staff schedules must be coordinated to ensure there are no disruptions in patient care.
Because some vaccine recipients experienced flu-like side effects in clinical trials, immunizations also must be staggered to ensure necessary staffing levels.
UCI Health began preparing for the arrival of the novel coronavirus early last February, long before it had spread widely in the United States.
The staff continued to refine its treatment plans, employee and patient safety protocols through the spring and summer as cases crested, receded and crested again.
Along the way, plans for an eventual vaccine roll out began, include the purchasing of more than 30,000 needles over the summer.
'Amazing science' led to the vaccines
At least one UCI employee, Lars Walton, chief of staff to UCI Chancellor Howard Gillman, was part of the clinical trial for the Pfizer vaccine — though he doesn’t know if he received the vaccine or a placebo.
Walton answered an ad to sign up for the trial, which was administered by Anaheim Clinical Trials, a clinical trials research center. He received the first dose in early September and a second dose three weeks later. He hasn’t had any unusual symptoms.
“A lot of people are nervous about it, and they’re reading 100-word tweets on it from people who don’t have a science background,” Walton says.
“But there’s a lot of amazing science behind this, and you can check out all the information on the FDA’s authorization. If you’re nervous, I would really recommend reading the FDA authorization rather than articles or social media posts written by people who aren’t medical professionals.”
The Pfizer trial is expected to last two years, and over that time, Walton will keep a weekly health diary and get blood drawn for analysis every three months. Trial participants who want can also leave the study to receive the vaccine.
“I think it’s amazing that your body can fix itself like this. When you break a bone, your body can heal. And with this mRNA vaccine, the vaccine is actually giving your body instructions on how to fight off the virus,” Walton says.
“This virus has prompted science to make a huge leap forward that we otherwise wouldn’t have been forced to make. That’s the silver lining of all this tragedy.”
Related stories
Explore further
Browse more blog posts by topic.






